Hydroxyl Radicals Reactivity
Hydroxyl free radicals OH. are an ultimate oxidation species — able to attack and oxidize molecules in their vicinity in order to balance their unpaired electron configuration.
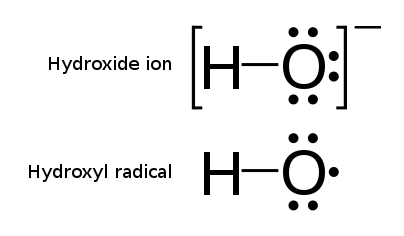
Hydroxyl or hydroxy groups (OH) form hydroxy anions OH-, which carry a negative charge. They become free radicals when one electron is removed from this configuration. As a result, the electric charge in hydroxyl radicals becomes balanced (neutral), but their electron spin configuration is unpaired, and therefore highly unstable. This feature makes the hydroxyl radical configuration extremely reactive towards oxidative reactions. The unpaired spin configuration forces the hydroxyl radical toward immediate capturing (or sharing) the missing electron from another molecule. As a result, the hydroxyl radicals are the ultimately powerful oxidation agents, capable of instantly oxidizing other molecules. The most consequential feature of the hydroxyl radicals is their very high oxidative potential (Eo) which is the highest of available oxidative agents (and second only to fluorine) which makes hydroxyl radicals the most powerful species in a multitude of oxidative reactions - as is illustrated in the Table below. With its supreme oxidation potential (significantly exceeding atomic oxygen species and much higher than ozone) hydroxyl radicals can access molecules and chemicals that are otherwise extremely stubborn and cannot be oxidated for example by the reactive oxygen, ozonation, by hydrogen peroxide alone or chlorine.
| Species | Oxidation potential [V] |
|---|---|
| Fluorine | 3.03 |
| Hydroxyl Radical | 2.80 |
| Atomic Oxygen | 2.42 |
| Ozone | 2.07 |
| Hydrogen Peroxide | 1.78 |
| Permanganate | 1.68 |
| Chlorine Dioxide | 1,58 |
| Chlorine | 1.36 |
| Oxygen molecule | 1.26 |
| Ref. source |
In general, hydroxyl radicals can induce an overwhelming variety of oxidative reactions. Hydroxyl radicals can balance their unpaired electron configuration through directly adding themselves (in the form of OH group) for example to unsaturated or double, triple bonds, such as C=C, C=O, S=O, N=N. Another possibility is a hydroxylation path (introduction of a hydroxyl group - OH- into various organic compounds). Alternative path involves formation of a various types of secondary radicals, according to the reaction: OH· + RH → H2O + R·. In practice, the multitude of the variations of such possible reactions in almost limitless.
The hydroxyl radicals can therefore effectively break double bonds (C=C, N=N), degrade hydrocarbons, cause epoxidation and aromatic ring opening, radical polymerization, formation of secondary radicals and many other types of reactions. The radicals have an extraordinary ability to engage in reactions resulting in degradation, polymerization, addition, cyclyzation, as well as initiation, substitution, insertion, rearrangement, or termination. In instances involving secondary radicals, hydroxyl radicals attack the molecules in their vicinity and capture their electrons, thus creating other types of radicals - resulting in a possible radical chain process. One example is when a freshly produced hydroxyl radical in methanol solution immediately forms a methyl radical, which can subsequently be part of the methylation reaction. Numerous scientific articles were published, which demonstrated that reactions which involve introduction of free radicals or which combine the use of a catalyst together with the generation of free radicals are thus enabled, faster and more efficient (e.g. see Ref.). 
Hydroxyl radicals are being produced by nature in the atmosphere by the reaction of excited atomic oxygen with water droplets. The use of ultraviolet radiation with peroxides is also an attainable path, resulting from the high energy of UV - for example when it is catalyzed with titanium dioxideTiO2, while attempting to employ the hydroxyl radicals in practical oxidative reactions.
The practical use of hydroxyl radicals in the oxidative chemistry requires a method representing a simple and reliable approach, capable of generating vast and controllable amounts of hydroxyl radicals, while avoiding the use of complicated machinery and without consuming excessive amounts of energy.

Such method was developed by Hydrogen Link, namely Catalytic Advanced Oxidation using our proprietary catalyst. It opens door to a multitude of practical oxidative ractions, while only using the "cleanest chemical" - hydrogen peroxide H2O2 - as a source of the hydroxyl radicals.
Read more : Catalytic Advanced Oxidation, Oxycatalyst
The OXYCATALYST - is a critical enabler for Catalytic Advanced Oxidation . It is a unique heterogeneous catalyst that re-routs the decomposition reaction of H2O2 into formation of hydroxyl radicals, as an intermediate stage of the H2O2 decomposition.
Hydroxyl radicals are extremely short-lived (their lifetime is of the order of a nanosecond) and therefore are very difficult to be directly detected in situ. However, their formation can be evidenced by reactions involving hydroxyl radicals indicators, which are uniquely able to demonstrate the hydroxyl radicals in action.
The following tests illustrate the hydroxyl radicals generation in reactions with the addition of hydrogen peroxide H2O2.
Methylene Blue: Oxycatalyst vs. Manganese Dioxide MnO2
In this experiment, the solution of Methylene Blue was treated with hydrogen peroxide catalyzed by two different catalysts: manganese dioxide (MnO2) and the Oxycatalyst (with the same amounts of the hydrogen peroxide and the catalysts). The picture below shows the comparison of three vials: the left vial shows the initial Methylene Blue solution with hydrogen peroxide, the middle vial had the MnO2 added and the right vial - the Oxycatalyst. The reaction of the decomposition of hydrogen peroxide in the vial with the Oxycatalyst proceeded much faster and it was finished within minutes. The reaction in the vial with manganese dioxide was much slower and it took 3 hours. The decomposition of H2O2,with manganese dioxide did not change the color of Methylene Blue, demonstrating that the reaction involved only formation of oxygen gas, which is not capable of affecting Methylene Blue. By contrast, the Methylene Blue solution in the right vial with the Oxycatalyst had been quickly made transparent without any residues of the blue color, thus evidencing the formation of hydroxyl radicals (of which this reaction is a sensitive indicator) [see Ref 1, Ref 2]

AZO DYES: Oxycatalyst vs. Manganese Dioxide

of the Azo (N=N) dye
Several catalyst are known to decompose hydrogen peroxide (such as manganese dioxide, silver, platinum etc,), but this type of decomposition mechanism involves mainly generation of oxygen. By contrast, the Oxycatalyst generates hydroxyl radicals, as shown in this test. A solution of a red-colored Azo Dye (Allura Red) was subjected to hydrogen peroxide addition - with a catalyst. The Oxycatalyst was added to the left-side vial and manganese dioxide was added to the right side vial. The hydrogen peroxide was decomposed with MnO2 with the generation of oxygen, but neither the hydrogen peroxide itself nor the formed oxygen species were able to remove the color of the dye, i.e. to affect the double bonds N=N. Only the hydroxyl radicals have the oxidation potential capable of oxidizing these bonds and thus capable of decoloration of the AZO DYES. The oxycatalyst caused full decoloration of the red dye very quickly thus evidencing the formation of hydroxyl radicals in this reaction.
Read more: Dye decoloration and degradation, Catalytic Advanced Oxidation, Oxycatalyst
Oxidation of Coumarin by hydroxyl radicals

Another example of the heterogeneous oxidation is shown below in a reaction of the oxidation of coumarin. Although coumarin is not soluble in hydrogen peroxide, when in contact with the catalyzed decomposition of H2O2 it undergoes instant hydroxylation.

This reaction is demonstrated by the formation of highly UV-fluorescent hydroxycoumarins (which can be observed under the UV light). If the reaction is carried out even further, full demineralization of coumarin can be achieved.
Degradation of Cellulose by hydroxyl radicals
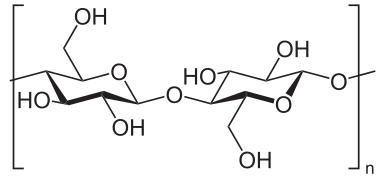
Cellulose is a compact, dense polysacharide which composes essential parts of the plants tissue, especially the structural parts that require strength and resilience, including plant cell walls, natural fibers and wood.

Cellulose is relatively resistant to degradation or decomposition, as compared to the softer plant tissue, especially because of the compactness and sturdiness of the cellulose formations. For this reason, the oxidation of cellulose (cellulolysis) i.e. hydrolysis of cellulose can be performed in extreme conditions - either in very high (alkaline) or very low (acidic) pH environment.

Therefore, it is extraordinary to be able to achieve oxidation of cellulose at neutral pH, without the need for highly acidic or alkaline conditions. It is however possible due to the hydroxyl radicals reactivity, where hydroxylation of cellulose can be done effectively.
Hydroxyl radicals are capable of full degradation of cellulose through a series of decomposition stages into more and more degraded stages.
Read more: Organic Matter degradation
The hydroxyl radicals are not selective in the sense that they are thermodynamically capable of oxdizing most of oxidizable molecules - because of their high oxidative potential. In practice however the reaction occurs along the paths that are most energetically favorable, i.e. reaction paths with the highest advantage of potential change. This means attacking preferentially strong reducing agents or the weakest bonds in the molecule, for example unsaturated bonds. Therefore, the reactions can be in practice highly controllable through the following:
- engineering of the reaction path by the choice of components to be exposed to the the hydroxyl reactivity (formation of other radicals, stepwise introduction of the components, chain reactions etc.)
- controlled rate of the formation of radicals (through the precise amount of the H2O2 and the catalyst to achieve the required amount of the radicals)
-
the use of radical sinks, scavengers and quenching of the reaction at any required moment
Please contact us with inquires related to cooperation and purchase of our catalysts: Contact for Inquiry or contact@hydrogenlink.com
EXAMPLES OF REACTIONS
Hydroxyl radicals are capable of full decomposition of cellulose through a series of decomposition stages into more and more degraded stages.

Degradation of Cellulose by Catalyzed Hydrogen Peroxide
Toxic chemical contaminants can be eliminated by the catalytic decomposition of hydrogen peroxide.
Phenol Degradation by Catalyzed Hydrogen Peroxide
Formation of the hydroxyl radicals is illustrated by the reaction with methylene blue — a hydroxyl radical indicator. Methylene Blue (methylthioninium chloride) is a sensitive indicator of the presence of hydroxyl radicals, because in the oxidative environment it can undergo discoloration only through the interaction with hydroxyl radicals. Otherwise, the decoloration of Methylene Blue is observed only in reduction reactions.
Methylene Blue discoloration evidencing the hydroxyl radicals formation
Read more
