Catalytic Oxygen Generation from Hydrogen Peroxide
Gaseous oxygen has a multitude of uses - from health supplementation (altitude sickness, breathing problems, blood circulation problems, and "well-being" or entertainment oxygen supplementation) to fish tank aeration, water aeration, agricultural and horticultural uses, as well as a variety of chemical reactions.

Hydrogen peroxide H2O2 is a very convenient, immediate source of oxygen for a variety of uses and at a wide scale of applications, from small household needs to larger scale oxygen generation.
The general reaction of the hydrogen peroxide decomposition results in the formation of water molecules and oxygen gas:
2 H2O2 → 2 H2O + O2
 In practice however, this reaction does not occur easily without a catalyst. Commercially available solutions of hydrogen peroxide (industrial, cosmetic and food grade) are always stabilized against decomposition and they contain chemicals that prevent formation of oxygen under normal conditions. This is why the solution of hydrogen peroxide can be stored for a prolonged period of time.
In practice however, this reaction does not occur easily without a catalyst. Commercially available solutions of hydrogen peroxide (industrial, cosmetic and food grade) are always stabilized against decomposition and they contain chemicals that prevent formation of oxygen under normal conditions. This is why the solution of hydrogen peroxide can be stored for a prolonged period of time.
There is however no easier generation of oxygen gas than Catalytic Oxygen Generation method with the catalyst developed by Hydrogen Link. The catalyst is in the form of a granulate (3-5 mm), which immediately induces decomposition of hydrogen peroxide with the release of gaseous oxygen, leaving behind clean water. In order to initiate the oxygen flow, simply pour some pharmacy grade hydrogen peroxide onto the catalyst granules. The reaction ceases when H2O2 is consumed and you can simply add the next portion of the hydrogen peroxide to your container. From time to time, the excess of water can be poured out from the container, in order to make room for the upcoming hydrogen peroxide feed. The feeding can be alternatively applied dropwise or as a continuous flow feed.
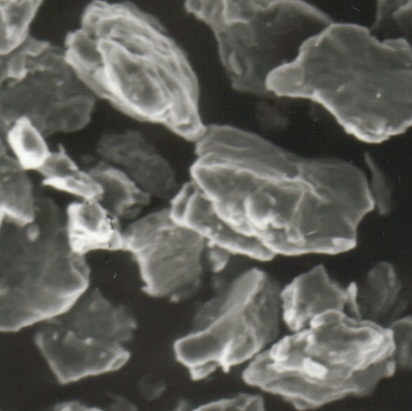
Catalysts can be very efficient in the decomposition of hydrogen peroxide. It is a fast and convenient method of producing oxygen, where high flow of oxygen can be easily and safely achieved. Higher concentrations of hydrogen peroxide on the other hand can be extremely powerful and can be used as a rocket propellant, for example.

Heterogeneous catalyst developed at Hydrogen Link has an additional advantage. It is solid and does not diissolve in the solution, therefore it can be reused many times with new portions of hydrogen peroxide, while the reaction product is simply clean water. Other advantages of our heterogeneous catalyst are very high efficiency (much higher than manganese dioxide or silver) and low cost .The presence of stabilizers in hydrogen peroxide does not impair the decomposition and any type and grade of H2O2 can be used

The Oxygen Generator using the Catalyst and hydrogen peroxide is very simple. It consists of a container / reactor (which may be made of plastic, glass or metal) and two plastic hoses - one inlet (dosing of hydrogen peroxide) and one for the oxygen outlet.
Please contact us with inquires related to cooperation and purchase of our catalysts: Contact for Inquiry or contact@hydrogenlink.com
“Solid” source of oxygen
Hydrogen peroxide is commonly available as a water solution, with concentrations ranging from 3% (household use) up to 50% (industrial concentration). However, the ballast of water in such solutions can become a disadvantage in some applications. In such cases, alternative, solid forms of hydrogen peroxide can be used, such as adducts of H2O2 with sodium carbonate or urea (i.e.sodium percarbonate and percarbamide)
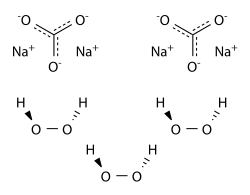
Sodium percarbonate (SPC) - Na2CO3·1.5H2O2 - is a crystalline adduct of H2O2 with sodium carbonate (“ash soda”). It is a convenient and concentrated source of hydrogen peroxide., easy to handle and transport. The amount of hydrogen peroxide by weight in sodium percarbonate exceeds 30%. It can be handled more conveniently and more safely than the same concentration of H2O2 in water solutions. Sodium percarbonate is used in many laundry detergents and cleaning products which are environmentally friendly, such as OxiClean and Vanish.
In water, sodium percarbonate releases hydrogen peroxide according to the reaction:
2Na2CO3.3H2O2 → 2Na2CO3 + 3H2O2
Hydrogen peroxide can be released from SPC into water, or into anhydrous solvents. However, in order to produce oxygen, the decomposition of thus released hydrogen peroxide still needs a catalyst, since this solution behaves similarly to the water-based hydrogen peroxide.
Catalytic decomposition of hydrogen peroxide from sodium percarbonate is equally efficient as from the water-based H2O2 – both as the source of oxygen and as the source of hydroxyl radicals, if needed for specialized chemical reactions.
Our heterogeneous (solid) catalyst can be used to generate oxygen from the solid sodium percarbonate (SPC) in a variety of reactions:
- Solid SPC + solid catalyst – can be conveniently transported and handled and subsequently combined with some water for oxygen generation
- Solid SPC can be first dissolved in water, and the solid catalyst added to the solution to generate oxygen.
Catalytic oxygen generation from Sodium percarbonate
The reaction proceeds until all hydrogen peroxide is consumed. Further portions of sodium percarbonate can be added to the same catalyst for more oxygen to be generated.
Sodium percarbonate is a very efficient source of oxygen. The amount of 30% of H2O2 in the solid form corresponds to a significant amount of released oxygen. For example, 10 g of catalyzed sodium percarbonate (a tablespoon) produces more than 1 liter of oxygen gas. Actually, more oxygen can be obtained from the catalyzed sodium percarbonate than from a pressurized (150 bars) gas cylinder of the same total weight (steel cylinder weight included). Moreover, sodium percarbonate can be used in portions as small as a "sachet" and it does not involve high pressures. The only byproduct of this reaction is ash soda (sodium carbonate) - a common and benign household product, sometimes used even in food.

Percarbamide (Carbamide Peroxide)
Another convenient solid source of hydrogen peroxide is carbamide peroxide (or percarbamide) which is an adduct of urea and hydrogen peroxide. It is a white odorless, non-toxic powder containing a large percentage of H2O2 - equivalent by weight to 36% concentration of hydrogen peroxide. Our heterogeneous catalyst enables efficient oxygen release form urea-hydrogen peroxide adduct and as such it can be used as a very convenient oxygen source or oxidative medium. The amount of produced oxygen is even higher than with the use of sodium percarbonate.
Catalyzed oxygen generation from hydrogen peroxide
click on the image to watch our video presentation
Please contact us with inquires related to cooperation and purchase of our catalysts: Contact for Inquiry or contact@hydrogenlink.com
Read more
